Atrial septal defect
| Atrial septal defect | |
|---|---|
| Classification and external resources | |
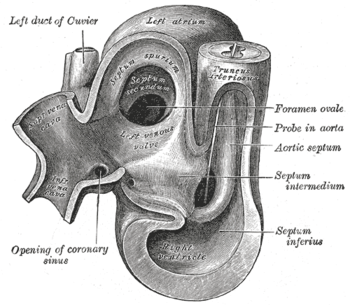 Heart of human embryo of about thirty-five days |
|
| ICD-10 | Q21.1 |
| ICD-9 | 745.5-745.6 |
| OMIM | 108800 |
| DiseasesDB | 1089 |
| eMedicine | med/3519 |
| MeSH | C14.240.400.560.375 |
Atrial septal defect (ASD) is a form of congenital heart defect that enables blood flow between the left and right atria via the interatrial septum. The interatrial septum is the tissue that divides the right and left atria. Without this septum, or if there is a defect in this septum, it is possible for blood to travel from the left side of the heart to the right side of the heart, or vice versa.[1] Irrespective of interatrial communication bi-directions, this results in the mixing of arterial and venous blood. The mixing of arterial and venous blood may or may not be hemodynamically significant, if even clinically significant. This mixture of blood may or may not result in what is known as a "shunt". The amount of shunting present, if any, dictates hemodynamic significance (see Pathophysiology below). A "right-to-left-shunt" typically poses the more dangerous scenario (see Pathophysiology below).
The right side of the heart contains venous blood with a low oxygen content, and the left side of the heart contains arterial blood with a high oxygen content. The construction of a heart void of an ASD prevents interatrial communication by means of an uncompromised interatrial septum. This prevents the atria from regular communication with each other, and thus oxygen-rich blood and oxygen-deficient blood do not mix together improperly.
During development of the fetus, the interatrial septum develops to eventually separate the left and right atria. The foramen ovale (pronounced /fɒˈreɪmən oʊˈvɑːli/) remains open during fetal development to allow blood from the venous system to bypass the lungs directly and enter the circulatory system. This is because the oxygen content of the fetal arterial system is provided by the placenta, as the lungs of the fetus are nonfunctional when it comes to oxygenizing the blood. A layer of tissue begins to cover the foramen ovale during fetal development, in which typically, after birth, the pressure in the pulmonary circulatory system drops, thus causing the foramen ovale to close entirely. In approximately 25% of adults{{[2]}}, the foramen ovale does not entirely seal[3]. In this case, elevation of pressure in the pulmonary circulatory system (i.e.: pulmonary hypertension due to various causes, or transiently during a cough) can cause the foramen ovale to remain open. This is known as a patent foramen ovale (PFO).
Contents |
Pathophysiology
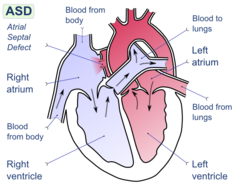
In unaffected individuals, the chambers of the left side of the heart make up a higher pressure system than the chambers of the right side of the heart. This is because the left ventricle has to produce enough pressure to pump blood throughout the entire body, while the right ventricle only has to produce enough pressure to pump blood to the lungs.
In the case of a large ASD (>9mm), which may result in a clinically remarkable left-to-right shunt, blood will shunt from the left atrium to the right atrium causing excessive interatrial communication (In the case of hemodynamically significant ASD (Qp:Qs > 1.5:1), the patient is often found to be notably symptomatic and ASD repair may be indicated). This extra blood from the left atrium may cause a volume overload of both the right atrium and the right ventricle, which if left untreated, can result in enlargement of the right side of the heart and ultimately heart failure.
Any process that increases the pressure in the left ventricle can cause worsening of the left-to-right shunt. This includes hypertension, which increases the pressure that the left ventricle has to generate in order to open the aortic valve during ventricular systole, and coronary artery disease which increases the stiffness of the left ventricle, thereby increasing the filling pressure of the left ventricle during ventricular diastole.
The right ventricle will have to push out more blood than the left ventricle due to the left-to-right shunt. This constant overload of the right side of the heart will cause an overload of the entire pulmonary vasculature. Eventually the pulmonary vasculature will develop pulmonary hypertension to try to divert the extra blood volume away from the lungs.
The pulmonary hypertension will cause the right ventricle to face increased afterload in addition to the increased preload that the shunted blood from the left atrium to the right atrium caused. The right ventricle will be forced to generate higher pressures to try to overcome the pulmonary hypertension. This may lead to right ventricular failure (dilatation and decreased systolic function of the right ventricle) or elevations of the right sided pressures to levels greater than the left sided pressures.
When the pressure in the right atrium rises to the level in the left atrium, there will no longer be a pressure gradient between these heart chambers, and the left-to-right shunt will diminish or cease.
If left uncorrected, the pressure in the right side of the heart will be greater than the left side of the heart. This will cause the pressure in the right atrium to be higher than the pressure in the left atrium. This will reverse the pressure gradient across the ASD, and the shunt will reverse; a right-to-left shunt will exist. This phenomenon is known as Eisenmenger's syndrome.
Once right-to-left shunting occurs, a portion of the oxygen-poor blood will get shunted to the left side of the heart and ejected to the peripheral vascular system. This will cause signs of cyanosis.
Epidemiology
As a group, atrial septal defects are detected in 1 child per 1500 live births. PFO are quite common (appearing in 10 - 20% of adults) but asymptomatic and therefore undiagnosed. ASDs make up 30 to 40% of all congenital heart disease that is seen in adults.[4]
The ostium secundum atrial septal defect accounts for 7% of all congenital heart lesions. This lesion shows a female preponderance, with a male : female ratio of 1:2.[5]
Types of atrial septal defects
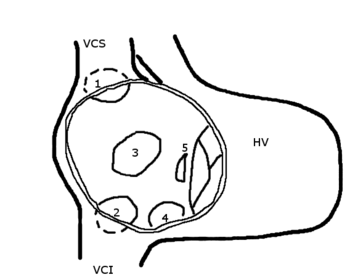
There are many types of atrial septal defects. They are differentiated from each other by whether they involve other structures of the heart and how they are formed during the developmental process during early fetal development.
Ostium secundum atrial septal defect
The ostium secundum atrial septal defect is the most common type of atrial septal defect, and comprises 6-10% of all congenital heart diseases.
The secundum atrial septal defect usually arises from an enlarged foramen ovale, inadequate growth of the septum secundum, or excessive absorption of the septum primum. Ten to twenty percent of individuals with ostium secundum ASDs also have mitral valve prolapse.[6]
Natural history
Most individuals with an uncorrected secundum ASD don't have significant symptoms through early adulthood. About 70% develop symptoms by the time they are in their 40s. Symptoms are typically decreased exercise tolerance, easy fatigueability, palpitations, and syncope.
Complications of an uncorrected secundum ASD include pulmonary hypertension, right-sided heart failure, atrial fibrillation or flutter, stroke, and Eisenmenger's syndrome.
While pulmonary hypertension is unusual before 20 years of age, it is seen in 50% of individuals above the age of 40. Progression to Eisenmenger's syndrome occurs in 5 to 10% of individuals late in the disease process.
Patent foramen ovale
A patent foramen ovale (PFO) is a small channel that has little hemodynamic consequence; it is a remnant of the fetal foramen ovale. Clinically it is linked to decompression sickness, paradoxical embolism and migraine. On echocardiography, there may not be any shunting of blood noted except when the patient coughs.
There is debate within the neurology and cardiology communities about the role of a PFO in cryptogenic (i.e. of unknown cause) neurologic events such as strokes and transient ischemia attacks (TIAs) without any other potential cause. Even though some data suggested that PFOs may be involved in the pathogenesis of some migraine headaches, this hypothesis has been disproved. Several clinical trials are currently underway to investigate the role of PFO in these clinical situations.
Ostium primum atrial septal defect
A defect in the ostium primum is occasionally classified as an atrial septal defect,[7] but it is more commonly classified as an atrioventricular septal defect.[8][9]
Sinus venosus atrial septal defect
A sinus venosus ASD is a type of atrial septum defect in which the defect in the septum involves the venous inflow of either the superior vena cava or the inferior vena cava.
A sinus venosus ASD that involves the superior vena cava makes up 2 to 3% of all interatrial communication. It is located at the junction of the superior vena cava and the right atrium. It is frequently associated with anomalous drainage of the right-sided pulmonary veins into the right atrium (instead of the normal drainage of the pulmonary veins into the left atrium).[10]
Common or single atrium
Common (or single) atrium is a failure of development of the embryologic components that contribute to the atrial septal complex. It is frequently associated with heterotaxy syndrome.[11]
Diagnosis
Diagnosis in children
Most individuals with a significant ASD are diagnosed in utero or in early childhood with the use of ultrasonography or auscultation of the heart sounds during physical examination.
Diagnosis in adults
Some individuals with an ASD will have undergone surgical correction of their ASD during childhood. The development of signs and symptoms due to an ASD are related to the size of the intracardiac shunt. Individuals with a larger shunt tend to present with symptoms at a younger age.
Adults with an uncorrected ASD will present with symptoms of dyspnea on exertion (shortness of breath with minimal exercise), congestive heart failure, or cerebrovascular accident (stroke). They may be noted on routine testing to have an abnormal chest x-ray or an abnormal ECG and may have atrial fibrillation.
Physical exam auscultation of the heart
The physical findings in an adult with an ASD include those related directly to the intracardiac shunt, and those that are secondary to the right heart failure that may be present in these individuals.
Upon auscultation of the heart sounds, there may be an ejection systolic murmur that is attributed to the pulmonic valve. This is due to the increased flow of blood through the pulmonic valve rather than any structural abnormality of the valve leaflets.
In unaffected individuals, there are respiratory variations in the splitting of the second heart sound (S2). During respiratory inspiration, the negative intrathoracic pressure causes increased blood return into the right side of the heart. The increased blood volume in the right ventricle causes the pulmonic valve to stay open longer during ventricular systole. This causes a normal delay in the P2 component of S2. During expiration, the positive intrathoracic pressure causes decreased blood return to the right side of the heart. The reduced volume in the right ventricle allows the pulmonic valve to close earlier at the end of ventricular systole, causing P2 to occur earlier.
In individuals with an ASD, there is a fixed splitting of S2. The reason why there is a fixed splitting of the second heart sound is that the extra blood return during inspiration gets equalized between the left and right atrium due to the communication that exists between the atria in individuals with ASD.
The right ventricle can be thought of as continuously overloaded because of the left to right shunt, producing a widely split S2. Because the atria are linked via the atrial septal defect, inspiration produces no net pressure change between them, and has no effect on the splitting of S2. Thus, S2 is split to the same degree during inspiration as expiration, and is said to be “fixed.”
Echocardiography
In transthoracic echocardiography, an atrial septal defect may be seen on color flow imaging as a jet of blood from the left atrium to the right atrium.
If agitated saline is injected into a peripheral vein during echocardiography, small air bubbles can be seen on echocardiographic imaging. It may be possible to see bubbles travel across an ASD either at rest or during a cough. (Bubbles will only flow from right atrium to left atrium if the RA pressure is greater than LA).
Because better visualization of the atria is achieved with transesophageal echocardiography, this test may be performed in individuals with a suspected ASD which is not visualized on transthoracic imaging.
Newer techniques to visualize these defects involve intracardiac imaging with special catheters that are typically placed in the venous system and advanced to the level of the heart. This type of imaging is becoming more common and involves only mild sedation for the patient typically.
If the individual has adequate echocardiographic windows, it is possible to use the echocardiogram to measure the cardiac output of the left ventricle and the right ventricle independently. In this way, it is possible to estimate the shunt fraction using echocardiograpy.
Transcranial Doppler (TCD) Bubble study
A less invasive method for detecting a PFO or other ASDs than transesophagal ultrasound is Transcranial Doppler with bubble contrast.[12] This method reveals the cerebral impact of the ASD or PFO.
Electrocardiogram
The ECG findings in atrial septal defect vary with the type of defect the individual has. Individuals with atrial septal defects may have a prolonged PR interval (a first degree heart block). The prolongation of the PR interval is probably due to the enlargement of the atria that is common in ASDs and the increased distance due to the defect itself. Both of these can cause an increased distance of internodal conduction from the SA node to the AV node.[13]
In addition to the PR prolongation, individuals with a primum ASD have a left axis deviation of the QRS complex while those with a secundum ASD have a right axis deviation of the QRS complex. Individuals with a sinus venosus ASD exhibit a left axis deviation of the P wave (not the QRS complex).
A common finding in the ECG is the presence of incomplete RBBB. In fact this finding is so characteristic that if it is absent, the diagnosis of ASD should be revised.
Treatment
Once someone is found to have an atrial septal defect, a determination of whether it should be corrected has to be made.
Surgical mortality due to closure of an ASD is lowest when the procedure is performed prior to the development of significant pulmonary hypertension. The lowest mortality rates are achieved in individuals with a pulmonary artery systolic pressure of less than 40 mmHg.
If Eisenmenger's syndrome has occurred, there is significant risk of mortality regardless of the method of closure of the ASD. In individuals who have developed Eisenmenger's syndrome, the pressure in the right ventricle has raised high enough to reverse the shunt in the atria. If the ASD is then closed, the afterload that the right ventricle has to act against has suddenly increased. This may cause immediate right ventricular failure, since it may not be able to pump the blood against the pulmonary hypertension.
Closure of an ASD in individuals under age 25 has been shown to have a low risk of complications, and individuals have a normal lifespan (comparable to a healthy age-matched population). Closure of an ASD in individuals between the ages of 25 and 40 who are asymptomatic but have a clinically significant shunt is controversial. Those that perform the procedure believe that they are preventing long-term deterioration in cardiac function and preventing the progression of pulmonary hypertension.
Methods of closure of an ASD include surgical closure and percutaneous closure.
Evaluation prior to correction
Prior to correction of an ASD, an evaluation is made of the severity of the individual's pulmonary hypertension (If present at all) and whether it is reversible (Closure of an ASD may be recommended for prevention purposes, to avoid such a complication in the first place. Pulmomary hypertension is not always present in adults that are diagnosed with an ASD in adulthood).
If pulmonary hypertension is present, the evaluation may include a right heart catheterization. This involves placing a catheter in the venous system of the heart and measuring pressures and oxygen saturations in the SVC, IVC, right atrium, right ventricle, pulmonary artery, and in the wedge position. Individuals with a pulmonary vascular resistance (PVR) of less than 7 wood units show regression of symptoms (including NYHA functional class). On the other hand, individuals with a PVR of greater than 15 wood units have increased mortality associated with closure of the ASD.
If the pulmonary arterial pressure is more than 2/3 the systemic systolic pressure, there should be a net left-to-right shunt of at least 1.5:1 or evidence of reversibility of the shunt when given pulmonary artery vasodilators prior to surgery. (If eisenmenger's physiology has set in, it must be proven that the right-to-left shunt is reversible with pulmonary artery vasodilators prior to surgery.)
Surgical ASD closure
Surgical closure of an ASD involves opening up at least one atrium and closing the defect with a patch under direct visualization.
Percutaneous ASD closure
Percutaneous closure of an ASD is currently only indicated for the closure of secundum ASDs with a sufficient rim of tissue around the septal defect so that the closure device does not impinge upon the SVC, IVC, or the tricuspid or mitral valves. The Amplatzer Septal Occluder (ASO) is commonly used to close ASDs. The ASO consists of two self-expandable round discs connected to each other with a 4 mm waist, made up of 0.004–0.005´´ nitinol wire mesh filled with Dacron fabric. Implantation of the device is relatively easy. The prevalence of residual defect is low. The disadvantages are a thick profile of the device and concern related to a large amount of nitinol (a nickel-titanium compound) in the device and consequent potential for nickel toxicity.
Percutaneous closure is the method of choice in most centres.[14]
Associated conditions
Due to the communication between the atria that occurs in ASDs, disease entities or complications from the condition, are possible.
Decompression sickness
ASDs, and particularly PFOs, are a predisposing risk factor for decompression sickness in divers because a proportion of venous blood carrying inert gases, such as helium or nitrogen does not pass through the lungs.[15][16] The only way to release the excess inert gases from the body is to pass the blood carrying the inert gases through the lungs to be exhaled. If some of the inert gas-laden blood passes through the PFO, it avoids the lungs and the inert gas is more likely to form large bubbles in the arterial blood stream causing decompression sickness.
Paradoxical emboli
Venous thrombi (clots in the veins) are quite common. Embolization (dislodgement of thrombi) normally go to the lung and cause pulmonary emboli. In an individual with ASD, these emboli can potentially enter the arterial system. This can cause any phenomenon that is attributed to acute loss of blood to a portion of the body, including cerebrovascular accident (stroke), infarction of the spleen or intestines, or even a distal extremity (i.e.: finger or toe).
This is known as a paradoxical embolus because the clot material paradoxially enters the arterial system instead of going to the lungs.
Migraine
Some recent research has suggested that a proportion of cases of migraine may be caused by patent foramen ovale. While the exact mechanism remains unclear, closure of a PFO can reduce symptoms in certain cases.[17][18] This remains controversial. 20% of the general population have a PFO, which for the most part, is asymptomatic. 20% of the female population have migraines. And, the placebo effect in migraine typically averages around 40%. The high frequency of these facts makes statistically significant relationships between PFO and migraine difficult (i.e., the relationship may just be chance or coincidence). In a large randomized controlled trial the higher prevalence of patent foramen ovale in migraine patients was confirmed, but migraine headache cessation was not more prevalent in the group of migraine patients that underwent closure of their patent foramen ovale.[19]
See also
- Atrioventricular septal defect
- Cardiac output
- Congenital heart disease
- Heart sounds
- Pulmonary hypertension
- Vascular resistance
- Pulmonary vascular resistance
- Ventricular septal defect
- Illnesses of Ariel Sharon
- Minimally Invasive Heart Surgery
References
- ↑ Atrial septal defect at Mount Sinai Hospital
- ↑ Robbins Basic Pathology, 8th Edition, p. 384
- ↑ Atrial Septal Defect in PediatriceMedicine
- ↑ Kaplan S (1993). "Congenital heart disease in adolescents and adults. Natural and postoperative history across age groups". Cardiol Clin 11 (4): 543–56. PMID 8252558.
- ↑ Feldt R, Avasthey P, Yoshimasu F, Kurland L, Titus J (1971). "Incidence of congenital heart disease in children born to residents of Olmsted County, Minnesota, 1950-1969". Mayo Clin Proc 46 (12): 794–9. PMID 5128021.
- ↑ Leachman R, Cokkinos D, Cooley D (1976). "Association of ostium secundum atrial septal defects with mitral valve prolapse". Am J Cardiol 38 (2): 167–9. doi:10.1016/0002-9149(76)90144-2. PMID 952260.
- ↑ "Atrial Septal Defect Types - Mayo Clinic". http://www.mayoclinic.org/atrial-septal-defect/types.html. Retrieved 2007-10-14.
- ↑ Fix, James D.; Dudek, Ronald W. (1998). Embryology. Baltimore: Williams & Wilkins. pp. 52. ISBN 0-683-30272-8.
- ↑ Q21.2
- ↑ Davia J, Cheitlin M, Bedynek J (1973). "Sinus venosus atrial septal defect: analysis of fifty cases". Am Heart J 85 (2): 177–85. doi:10.1016/0002-8703(73)90458-4. PMID 4569755.
- ↑ Valdes-Cruz LM, Cayre RO (1998). Echocardiographic diagnosis of congenital heart disease. Philadelphia.
- ↑ Glen, S.; J. Douglas. (1995). "Transcranial doppler monitoring. (letter to editor)". South Pacific Underwater Medicine Society journal 25 (2). ISSN 0813-1988. OCLC 16986801. http://archive.rubicon-foundation.org/6409. Retrieved 2008-04-06.
- ↑ Clark E, Kugler J (1982). "Preoperative secundum atrial septal defect with coexisting sinus node and atrioventricular node dysfunction". Circulation 65 (5): 976–80. PMID 7074763.
- ↑ Bjørnstad P (2006). "Is interventional closure the current treatment of choice for selected patients with deficient atrial septation?". Cardiol Young 16 (1): 3–10. doi:10.1017/S1047951105002027. PMID 16454871.
- ↑ Lier H, Schroeder S, Hering R (2004). "[Patent foramen ovale: an underrated risk for divers?]". Dtsch Med Wochenschr 129 (1-2): 27–30. doi:10.1055/s-2004-812652. PMID 14703578.
- ↑ Saary M, Gray G (2001). "A review of the relationship between patent foramen ovale and type II decompression sickness". Aviat Space Environ Med 72 (12): 1113–20. PMID 11763113.
- ↑ Adams H (2004). "Patent foramen ovale: paradoxical embolism and paradoxical data". Mayo Clin Proc 79 (1): 15–20. doi:10.4065/79.1.15. PMID 14708944.
- ↑ Azarbal B, Tobis J, Suh W, Chan V, Dao C, Gaster R (2005). "Association of interatrial shunts and migraine headaches: impact of transcatheter closure". J Am Coll Cardiol 45 (4): 489–92. doi:10.1016/j.jacc.2004.09.075. PMID 15708691.
- ↑ "Migraine Intervention With STARFlex Technology (MIST) trial: a prospective, multicenter, double-blind, sham-controlled trial to evaluate the effectiveness of patent foramen ovale closure with STARFlex septal repair implant to resolve refractory migraine headache. - Dowson A et al. 117 (11): 1397-1404 - Circulation". http://circ.ahajournals.org/cgi/content/full/117/11/1397. Retrieved 2008-10-26.
|
||||||||||||||||||||||||||||||||||||